
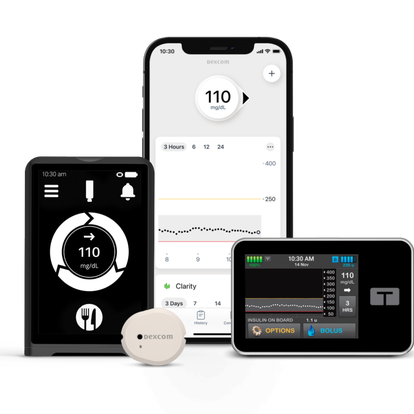
The Dexcom G7
Make better decisions in the moment with the most accurate CGM1
Manage your diabetes with confidence.
The wait is over.
Dexcom G7 now integrates with the Tandem t:slim X2TM insulin pump and Beta Bionics iLet Bionic Pancreas.
Expanded Medicare coverage
Due to recent Medicare changes, millions more people are now covered for Dexcom CGM. Dexcom G7 is the most accurate CGM system1 covered by Medicare*—and it’s easy to use and get started!2
No other CGM system is more affordable than Dexcom G7 for Medicare patients.†
Learn more and get a free benefits check to see if you qualify.
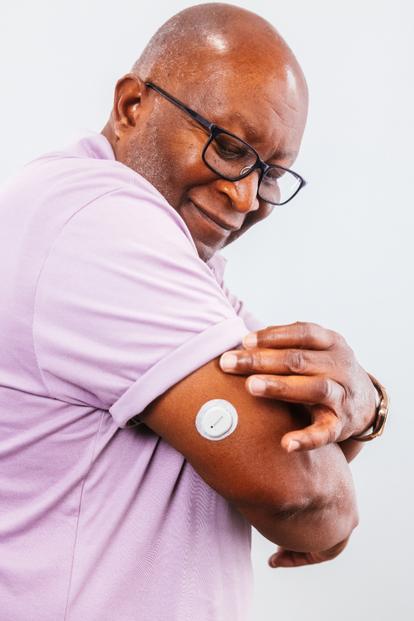
Image shown does not include required overpatch. Please be sure to follow instructions for using the overpatch.
The Dexcom CGM difference
As a pioneer and leader in real-time continuous glucose monitoring (RT-CGM), Dexcom helps to simplify diabetes management. Our CGM Systems provide best-in-class accuracy1 and exceptional convenience, allowing you to live a healthier, more confident life.
Smart device sold separately.‡

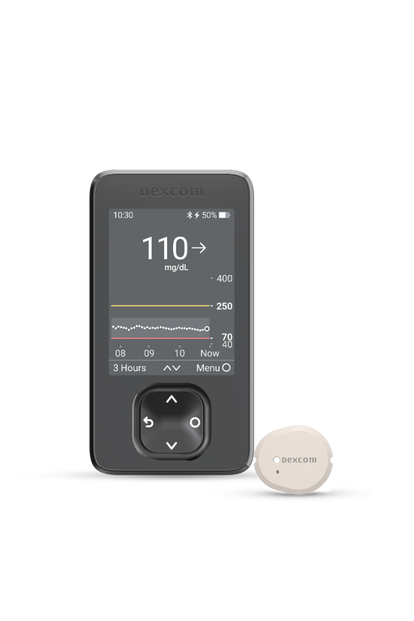
Real-time glucose readings
No fingersticks.§ No scanning.
§ Fingersticks required for diabetes treatment decisions if symptoms or expectations do not match readings.
No fingersticks.§ No scanning.
§ Fingersticks required for diabetes treatment decisions if symptoms or expectations do not match readings.
Easy to use
Easy to apply, easy to set up, and easy to use.2
Easy to apply, easy to set up, and easy to use.2
Exceptional accuracy
Dexcom offers the most accurate CGM system available.1
Dexcom offers the most accurate CGM system available.1
Proven results
Lower A1C3-7. More time in range.3-7
Lower A1C3-7. More time in range.3-7
Smart device sold separately.‡
Dexcom is the #1 recommended CGM brand by people with diabetes and healthcare providers.8,9
Image shown does not include required overpatch. Please be sure to follow instructions for using the overpatch.
Smart device sold separately.‡
Smart device sold separately.‡
Find the right Dexcom CGM System for you
Wherever you are in your diabetes journey, we have a RT-CGM to fit your needs. See our products comparison to learn more.

Dexcom G7
For those looking for the most accurate CGM system1 that’s also our easiest to use.

Dexcom G6
For those looking to connect their CGM system to an automated insulin delivery system, such as a connected pump.||

Dexcom is the #1 covered CGM brand10
Most covered patients pay $20 or less per month for Dexcom CGM¶,11
Image shown does not include required overpatch. Please be sure to follow instructions for using the overpatch.

Get started with Dexcom G7
If you are a new Dexcom user, fill out the Get Started form to get a free benefits check.
If you are a current Dexcom G6 user, please speak to your doctor about getting a prescription for Dexcom G7. Check out the FAQ for further details.
Our experts are here to help.
*Medicare covers Dexcom CGM for patients who meet the Medicare coverage criteria. For a list of Medicare coverage criteria, please visit the Center for Medicare and Medicaid services website. †Under Medicare’s DME fee schedule, reimbursement and coinsurance for CGM’s using CPT codes A4239 and E2103, are the same, regardless of CGM brand. ‡Smart device sold separately. To view a list of compatible devices, visit dexcom.com/compatibility. ||To learn more about insulin pump integrations and compatibility with Dexcom CGM Systems, visit dexcom.com/integrate. ¶Individual pricing may vary depending on insurance coverage. #Individual results may vary.
1 Dexcom, data on file, 2023. 2 Dexcom G7 CGM System User Guide, 2021. 3 Beck RW, et al. JAMA. 2017;317(4):371-378. 4 Beck RW, et al. Ann Intern Med. 2017;167(6):365-374. 5 Martens T, et al. JAMA. 2021;325(22):2262-2272. 6 Laffel LM, et al. JAMA. 2020;323(23):2388-2396. 7 Welsh JB, et al. J Diabetes Sci Technol. 2022:19322968221099879. 8 dQ&A US Diabetes Connections Patient Panel Report, Q1 2023 9 Seagrove HCP Survey Q2 2022. 10 Managed Markets Insights & Technology, LLC MMIT. 11 Dexcom, data on file, 2023.
BRIEF SAFETY STATEMENT: Failure to use the Dexcom Continuous Glucose Monitoring System and its components according to the instructions for use provided with your device and available at https://www.dexcom.com/safety-information and to properly consider all indications, contraindications, warnings, precautions, and cautions in those instructions for use may result in you missing a severe hypoglycemia (low blood glucose) or hyperglycemia (high blood glucose) occurrence and/or making a treatment decision that may result in injury. If your glucose alerts and readings from the Dexcom CGM do not match symptoms, use a blood glucose meter to make diabetes treatment decisions. Seek medical advice and attention when appropriate, including for any medical emergency.
MAT-1628
